Travis Baker, Assistant Professor at the Center for Molecular and Behavioral Neuroscience (CMBN), recently won a $2.5 million grant (UG3DA054787) from the National Institute on Drug Abuse (NIDA), part of the National Institutes of Health (NIH), to develop new brain-based interventions to correct aberrant reward processes that sustain substance-use disorders.
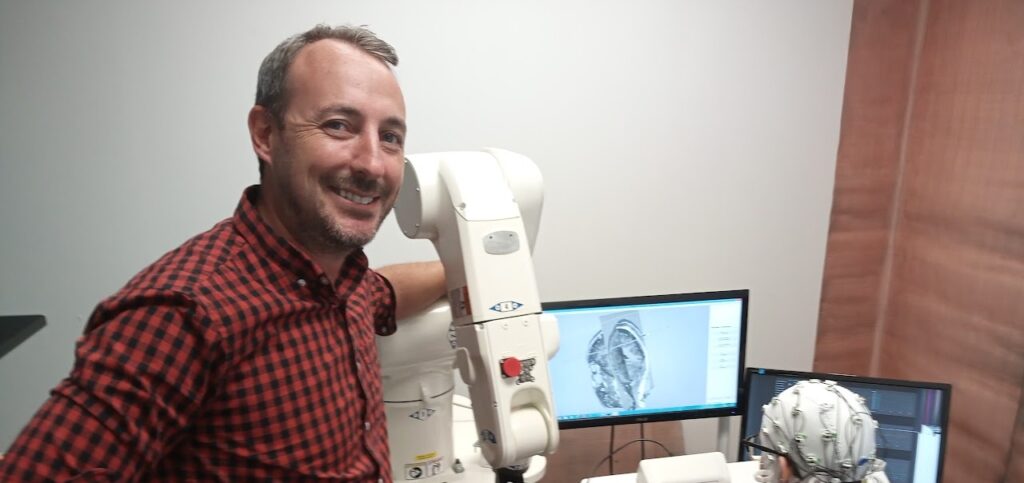
The grant, which covers two phases of research over five years, will support Baker’s use of a cutting-edge robot-assisted brain-imaging technology called transcranial magnetic stimulation (Ri-TMS) to induce electrical currents inside the brain with great precision to alter reward circuitry. It could potentially complementing current drug regimens to help treat nicotine and other addictions.
“I’d been trying for this grant for three years and was really happy that NIDA is now in a place to fund this type of research,” said Baker. “TMS has been successful in treating depression and has potential for addiction as well.”
Scientists have long known about the brain’s reward system, a network of regions and pathways that are activated with the release of neurotransmitters such as dopamine and seratonin when experiencing pleasurable stimuli. Dopamine, in particular, is associated with reward and motivation, with various pathways involved in causing us to memorize and want to repeat an activity that provides a rewarding sensation. The dopamine system also conveys a teaching signal to help the brain learn how to make better decisions or even, say, learn to ride a bike.
With drugs like cocaine, opiates or nicotine, the brain is triggered to release large amounts of dopamine, overstimulating the reward system and causing the brain to overlearn the behavior: In effect, the brain becomes oversensitive to cues that would predict drug-related rewards or behaviors, hampering our ability to make sound, healthy decisions.
“Drugs of abuse drive the dopamine signaling strongly, oversensitizing us to drug-related cues while at the same time desensitizing the system to natural rewards like going to work or school, or maintaining financial security, relationships or health,” said Baker. “Those goals get pushed to the side as goals for the drugs become highly salient, so there’s some bias in the decision-making system that’s introduced. The system becomes disregulated.”
With this grant, Baker is looking not to treat symptoms like drug tolerance, craving and withdrawal but to actually measure and modulate the reward-system circuitry in the brain to affect goal-directed behavior.
He’ll start by recording and measuring subjects’ neural activity as they’re engaged in decision-making or reward tasks, using a technique called electroencephalography (EEG), which involves electrodes placed on their scalp and measuring “reward positivity,” a biomarker generated in the anterior singular cortex (ASC), which is involved in goal-directed behavior, and which indicates when the brain processes a reward and how much value it attributes to it.
“The ASC uses dopamine signals to learn the value of rewards and what behaviors to select to obtain that reward,’ said Baker. “My earlier research has found that when you compare the reward positivity of a typical non-drug-using person with someone who has a substance-abuse disorder like nicotine or some other drug, the reward positivity to money rewards is abolished in the latter.”
To modulate the reward circuitry, Baker will use robot-assisted transcranial magnetic stimulation (Ri-TMS), which involves suspending a large magnetic coil outside the skull and inducing electrical currents inside brain the size of a dime to stimulate or inhibit parts of the brain it comes in contact with. The robotics guide and hold the coil in place and increase TMS’ precision exponentially as he continues to measure brain activity using EEG. Baker has already had some success using TMS to modulate reward activity in the ACS, but the process is highly technical, and this grant will help him hone his method to optimize the brain responses he’s looking for.
That’s phase 1 of the grant. In phase 2, Baker will employ this method as proof of concept with smokers, applying the stimulation protocol as their playing task for, say, monetary rewards, and applying the inhibitory protocol while they’re playing a task for drug-related rewards.
“With smokers, the reward system is biased toward drug-related rewards, compared to monetary or natural rewards,” said Baker, “so I’m trying to use this intervention to flip that in the brain, to reverse that bias.”
Baker cautions that this method is no one-shot wonder. He says the technique needs to be applied with other remedies for an effective drug-treatment program, yet is optimistic that, one day, TMS can be part of an effective treatment plan.
“Here I’m trying to treat the brain and get it to work like it used to. At the same time, individuals with substance-abuse disorder should maintain current treatment programs while doing this,” said Baker. “Because we’re trying to modulate the goal-directed systems, I’m hoping that this will allow individuals to have more control over their drug-related behavior, maintain treatment goals and achieve broader life goals. The next generation of brain therapies are using these brain-modulation techniques to identify ways to recover this reward function. So, it holds promise.”
Date: November 30, 2022
Media Contact: Carrie Stetler, cjs281@rutgers.edu